

Over the last 2 decades, the Diabetic Retinopathy Clinical Research (DRCR) Retina Network has completed more than 30 multicenter studies in diabetic retinopathy (DR) in collaboration with more than 160 participating clinical sites throughout the United States and Canada. These studies have played a pivotal role in advancing the care of diabetic eye diseases. For example, DRCR Retina Network studies helped establish anti-VEGF therapy as a first-line treatment for vision-threatening center-involving diabetic macular edema (CI-DME) and an effective alternative to panretinal photocoagulation (PRP) for the treatment of proliferative DR (PDR).
In recent years, the DRCR Retina Network has expanded its studies to include other retinal diseases beyond DR. Here is a look at the DRCR Retina Network’s recently published and ongoing trials.
Protocol T compared aflibercept (Eylea, Regeneron), bevacizumab (Avastin, Genentech/Roche), and ranibizumab (Lucentis, Genentech/Roche) for eyes with visual impairment due to CI-DME (Figure 1). At the end of the 2-year study period, there were no differences between the agents with regards to the mean change in VA from baseline, if baseline VA was between 20/32 and 20/40. However, among patients with a baseline VA between 20/50 and 20/320, aflibercept was superior to bevacizumab and ranibizumab at 1 year, and to bevacizumab at 2 years. 1
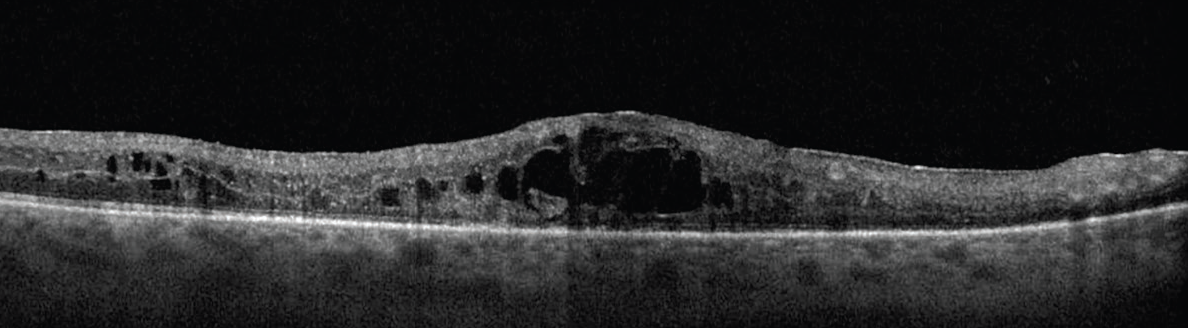
Click to view larger
Figure 1. The DRCR Retina Network studies were integral to the shift toward anti-VEGF as the first-line treatment for CI-DME, as seen here.
Patients from Protocol T were asked to follow up for a single visit at 5 years as part of the Protocol T extension study, Protocol TX, to assess clinical outcomes. A total of 317 of 463 patients completed the 5-year follow-up; 68% received at least one anti-VEGF injection between years 2 and 5 (median [range]: 4 [0-12]). The mean VA at 5 years improved by 7.4 letters from baseline but was 4.7 letters less than year 2. Overall, mean central subfield thickness (CST) stayed stable between years 2 and 5. 2
Protocol V evaluated three treatment strategies for patients with good vision (20/25 or better) and CI-DME: intravitreal aflibercept as frequently as every 4 weeks, focal/grid laser therapy with deferred aflibercept, or observation with deferred aflibercept. The primary outcome was at least a 5-letter VA decrease from baseline at 2 years. 3
At 2 years, there was no statistically significant difference in the percentage of patients who lost at least 5 letters between the three groups, whether they were initially treated with aflibercept or received aflibercept as a rescue when visual acuity worsened, for those assigned to the laser or observation groups. The researchers concluded that observation may be a reasonable initial strategy for patients with good vision and CI-DME until visual acuity worsens. 3
In a post-hoc analysis of Protocol V’s observation group, 80 of 236 (34%) patients received treatment with aflibercept during the 2-year follow-up. Patients with a CST ≥ 300 μm, more severe DR stage, or fellow non-study eye receiving treatment for DME within 4 months of randomization were more likely to receive rescue treatment with aflibercept. Eyes that were treated with initial observation and then with aflibercept if visual acuity worsened maintained good vision at 2 years. 4
Protocol W evaluated the effect of intravitreal anti-VEGF aflibercept versus sham for the prevention of vision-threatening complications of moderate to severe nonproliferative DR (NPDR). Patients were randomly assigned to receive intravitreal aflibercept injection or sham at baseline, at months 1, 2, and 4, and then every 4 months through 2 years. Between years 2 and 4, aflibercept was administered if patients developed CI-DME with vision loss or high-risk PDR. The primary outcome of the study was the development of vision-threatening CI-DME or PDR. 5
At 2 years, the cumulative probability of developing PDR was 13.5% and 33.2% and CI-DME with vision loss was 4.1% and 14.8% in the aflibercept and sham groups, respectively. Although periodic treatment with aflibercept reduced the development of vision-reducing CI-DME and PDR, it did not have any statistically significant visual acuity benefits at the end of 2 years. 5 The 4-year results will be revealed near the end of 2022 and will give us long-term assessment of whether prevention of vision-threatening CI-DME and PDR with aflibercept results in long-term visual benefits. 5
Protocol AA compared ultra-widefield (UWF) imaging with Early Treatment Diabetic Retinopathy Study (ETDRS) 7-standard-field imaging for the assessment of peripheral lesions, DR severity, and rates of DR worsening over time. This 4-year study evaluated the association of retinal nonperfusion on UWF fluorescein angiography (FA) with DR severity and predominantly peripheral lesions (Figure 2). The study concluded that 70% of the nonperfusion in diabetic eyes involves the peripheral retina and suggested that UWF-FA may better predict progression of DR compared with 7-standard-fields imaging, as increased nonperfusion on UWF-FA is associated with the presence of predominantly peripheral lesions. 6
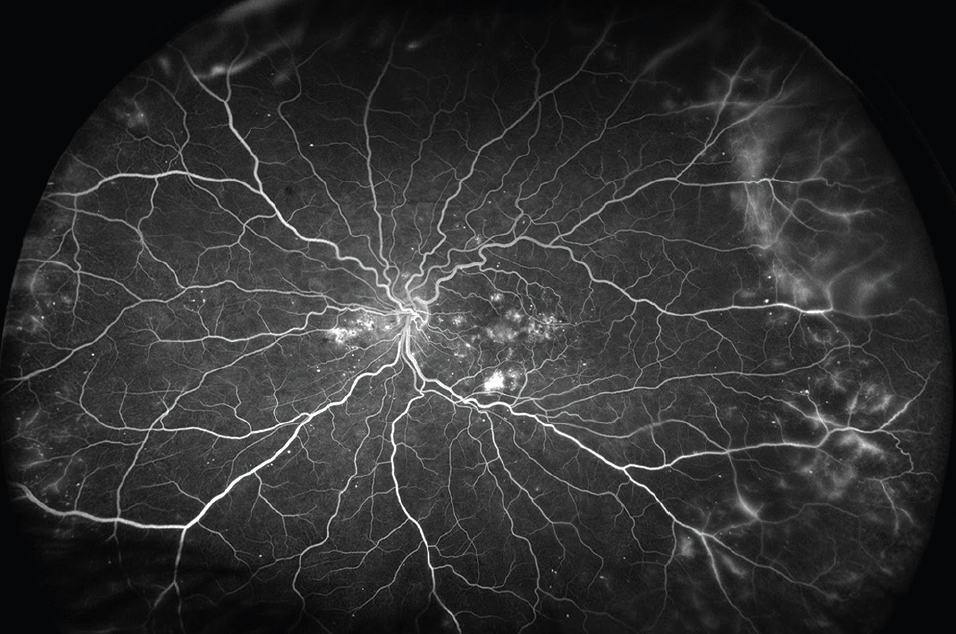
Click to view larger
Figure 2. UWF-FA imaging, as seen here, may better predict progression of DR compared with 7-standard-fields imaging, according to DRCR Retina Network’s Protocol AA.
Protocol AB compared the initial treatment for vitreous hemorrhage from PDR with intravitreal aflibercept versus vitrectomy with PRP. Patients were randomly assigned to aflibercept (4 monthly injections) or vitrectomy with PRP. The primary outcome was mean visual acuity at 24-weeks and the secondary outcome was mean visual acuity at 2 years. 7 There was no statistically significant difference in the mean visual acuity at 24 weeks or 2 years between the two groups. Over 2 years, 33% of patients in the aflibercept group required vitrectomy and 32% in vitrectomy with PRP group received subsequent aflibercept injections. The researchers concluded that the study may have been underpowered to detect a clinical benefit in favor of initial vitrectomy with PRP. 7
Protocol AC compared the efficacy of intravitreal aflibercept monotherapy versus intravitreal bevacizumab first with a switch to aflibercept beginning at week 12 if protocol-specific criteria were met in eyes with visual impairment (BCVA between 20/50 and 20/320) from CI-DME. The study concluded that there was no significant difference in visual outcomes over 2 years in eyes treated with aflibercept monotherapy versus those switched from bevacizumab to aflibercept due to suboptimal clinical response. It was suggested that initiating treatment with bevacizumab and switching to aflibercept is a safe and effective alternative to aflibercept monotherapy in diabetic eyes with moderate visual impairment from CI-DME. 8
This study created a real-world scenario to investigate the cost burden for patients and insurance companies and demonstrated that switching from bevacizumab to aflibercept when needed is an economical option, while still seeing visual outcomes similar to those seen when starting aflibercept from the outset, in a population similar to patients enrolled in the study. 8
Protocol AE was a phase 2 clinical trial that randomized patients to a home-based photobiomodulation (PBM) device versus placebo for CI-DME and good vision to see if this could be considered as a potential cost-effective treatment and whether a phase 3 trial would be warranted. The primary outcome was a change in CST on spectral-domain OCT at 4 months. The study concluded that, although photobiomodulation is safe and well-tolerated, it was not effective for reducing CST in eyes with CI-DME with good vision. 9
Protocol AF is a randomized, double-masked, placebo-controlled trial that will evaluate the effect of fenofibrate compared with placebo for the prevention of worsening of DR over 4 years of follow-up in eyes with mild to moderately severe NPDR and no CI-DME at baseline. The study is currently enrolling participants. 10
In 2018, the DRCR Retina Network expanded its scope to all retinal pathologies in a collaborative research setting. The first two non-DR protocols included protocols AG and AH.
Protocol AG compared pneumatic vitreolysis (PVL) with clinic-based injection of C3F8 versus sham injection for vitreomacular traction (VMT) without macular hole. 11
Protocol AH was a single-arm study that evaluated PVL with clinic-based injection of C3F8 for full-thickness macular hole associated with VMT. 11
The main outcome was central VMT release at 24 weeks for Protocol AG and macular hole closure at 8 weeks for Protocol AH. Both of these studies were terminated early due to higher-than-expected rates of retinal tears and retinal detachments. 11
Protocol AM is an ongoing trial that will compare visual acuity and OCT outcomes, changes in metamorphopsia, and complication rates at 36 months in eyes that undergo immediate versus deferred surgery for symptomatic epiretinal membranes. 12
Since 2002, the DRCR Retina Network has made substantial contributions to the way retina specialists manage diabetic eye disease, both medically and surgically. Now with an expanded scope of research to include all retinal pathologies, the DRCR Retina Network will continue to guide retina practices worldwide.
1. Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351-1359.
2. Glassman AR, Wells JA, Josic K, et al. Five-year outcomes after initial aflibercept, bevacizumab, or ranibizumab treatment for diabetic macular edema (Protocol T Extension Study). Ophthalmology. 2020;127(9):1201-1210.
3. Baker CW, Glassman AR, Beaulieu WT, et al. Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: a randomized clinical trial. JAMA. 2019;321(19):1880-1894.
4. Glassman AR, Baker CW, Beaulieu WT, et al. Assessment of the DRCR Retina Network approach to management with initial observation for eyes with center-involved diabetic macular edema and good visual acuity: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2020;138(4):341-349.
5. Maturi RK, Glassman AR, Josic K, et al. Effect of intravitreous anti-vascular endothelial growth factor vs sham treatment for prevention of vision-threatening complications of diabetic retinopathy: The Protocol W Randomized Clinical Trial. JAMA Ophthalmol. 2021;139(7):701-712.
6. Silva PS, Liu D, Glassman AR, et al. Assessment of fluorescein angiography nonperfusion in eyes with diabetic retinopathy using ultrawide field retinal imaging. Retina. 2022;42(7):1302-1310.
7. Antoszyk AN, Glassman AR, Beaulieu WT, et al. Effect of intravitreous aflibercept vs vitrectomy with panretinal photocoagulation on visual acuity in patients with vitreous hemorrhage from proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2020;324(23):2383-2395.
8. Jhaveri CD, Glassman AR, Ferris FL, et al. Aflibercept monotherapy or bevacizumab first for diabetic macular edema [Preprint published online July 14, 2022]. N Engl J Med.
9. Kim JE, Glassman AR, Josic K, et al. A randomized trial of photobiomodulation therapy for center-involved diabetic macular edema with good visual acuity (Protocol AE). Ophthalmol Retina. 2022;6(4):298-307.
10. Fenofibrate for prevention of DR worsening (Protocol AF). Accessed June 10, 2022. clinicaltrials.gov/ct2/show/NCT04661358
11. Chan CK, Mein CE, Glassman AR, et al. Pneumatic vitreolysis with perfluoropropane for vitreomacular traction with and without macular hole: DRCR Retina Network protocols AG and AH. Ophthalmology. 2021;128(11):1592-1603.
12. Randomized trial comparing immediate vs. deferred surgery for symptomatic ERM (Protocol AM). Accessed June 10, 2022. clinicaltrials.gov/ct2/show/NCT05145491
Saleema Kherani, MD, MPH
Ophthalmology Resident, Medical College of Wisconsin, Milwaukee
Financial disclosure: None
Judy E. Kim, MD, FARVO, FASRS
Professor of Ophthalmology, Medical College of Wisconsin, Milwaukee
Director, Teleophthalmology and Research, Medical College of Wisconsin, Milwaukee
Jekim@mcw.edu
Financial disclosure: Advisory Board (Alimera Science, Allergan/Abbvie, Apellis, Astella, Bausch + Lomb, Clearside, DORC, Genentech/Roche, Outlook Therapeutics, Notal Vision, Novartis, Regeneron)